*** Advanced Project Notice ***
The following project is an ADVANCED project and may require handling of dangerous materials and/or equipment and is intended to be conducted by adults only!
Purpose
To demonstrate the catalytic activity of Diastase enzyme.
Additional information
Enzymes are proteins. They are found in almost all living systems; even in some viruses. Millions of anabolic and catabolic reactions take place in living organisms. Usually these reactions can’t proceed, if we try to perform them externally in a temperature and pressure similar to that in living cell. A huge amount of energy is necessary to perform such reactions in-vitro (outside the cell). But strangely the living cell can perform these reactions in a very low temperature/pressure. How is it possible?
The secret is enzymes. They act as catalysts for in-vivo (inside cell) reactions. A catalyst can reduce the reaction energy by taking part in such reaction. At the end, reactants convert into products but the enzyme remains unmodified.
A diastase is any one of a group of enzymes which catalyses the breakdown of larger starch (a carbohydrate) molecules into rather small maltose units. Today, diastase means any a-, ß-, or ?-amylase that can break down carbohydrates. Diastases are commonly found in germinating seeds and in the digesting system of animals.
Sponsored Links
Required materials
- Germinating green gram seeds
- Mortar and pestle
- Muslin cloth
- Water bath maintained in 37ºC
- Thermometer
- Stop watch
- White tile
- 5ml measuring cylinder
- 1ml pipette
- Pipette bulb
- Droppers
- Balance
- Distilled water
- Starch solution
Starch is insoluble in water. Therefore to prepare a starch solution, dissolve 2g starch and 0.2 g salicylic acid in 100mL hot distilled water.
- Iodine solution
Lugol's iodine solution consists of 5 g iodine (I2) and 10 g potassium iodide (KI) mixed with 85 ml distilled water.
Estimated Experiment Time
About 1-2 hours
Step-By-Step Procedure
- 1. Weigh about 5g of germinating green gram seeds. Put into the mortar. Crush it with pestle.
- 2. Add about 10ml of distilled water and mix thoroughly.
- 3. Filter the solution through a muslin cloth to remove larger debris.
- 4. Take two test tubes. Label one test tube as “CONTROL” and the other as “EXP”. Put 15ml of starch solution in to each test tube.
- 5. Pour 5ml of the enzyme extraction (crushed green gram solution) into another test tube. Label it as “ENZ”.
- 6. Place all three test tubes in a water bath maintained in 37ºC.
- 7. Measure the temperature of starch solutions and enzyme solution. When the temperature of all solutions reach 37ºC, measure 1ml of enzyme solution into a pipette and pour it into the starch solution labeled as “EXP”. Mix with a glass rod.
- 8. As soon as you mix the enzyme solution with starch in “EXP” tube, activate the stop watch.
- 9. At time zero (as soon as you mix the enzyme solution with starch in “EXP” tube) take a drop out from each of the two solutions in tubes “EXP” and “CONTROL”
- 10. Put the starch drops separately on a white tile. Put a drop of iodine on each drop (using another dropper) and mix. Observe the color change. (Refer to Figure 1. in the Notes section below)
- 11. After each 5 minutes take a drop out from the starch-enzyme mixture and repeat step 10. Do the same with the starch solution in “CONTROL” tube.
- 12. Continue this for about one hour.
- 13. Tabulate your results as follow. (Refer to Figure 2. in the Notes section below)
Note
The controlled tube has same conditions as the other tube but contains only the substrate (starch). Controlled test can give a clue whether starch is subjected to any other reaction, under atmospheric pressure and given temperature, without the participation of enzyme solution.
Figures & Illustrations
| Figure 1 |
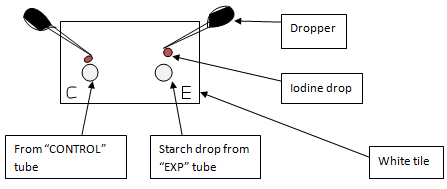 |
|
| Figure 2 |
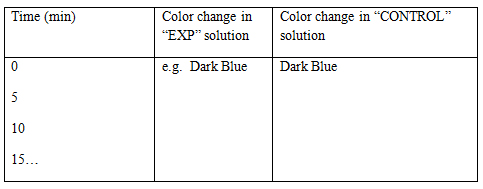 |
Observation
At time zero, you will see both drops from solutions in “CONTROL” and “EXP” tubes turn into dark blue color with the addition of iodine solution. After a certain period of time, the solution in “EXP” tube will not turn into dark blue when iodine is added.
Result
Iodine test is the identification test for starch. In the presence of iodine a starch solution will turn into dark blue color. The amylose, or straight chain portion of starch, forms helices where iodine molecules assemble, these helices are responsible for the dark blue color.
The extraction from germinating green grams is rich in diastase (amylases) enzymes. When starch solution is mixed with the enzyme solution, larger starch molecules break down into smaller fragments. Therefore at a certain point the starch-enzyme mixture would not give positive results for iodine test. This indicates that there are no more starch molecules remaining in the solution.
In controlled test, the starch solution will keep giving positive result for the iodine test. That is because in given temperature/pressure the starch molecules can’t break into smaller units spontaneously.
Sponsored Links
Take a moment to visit our table of Periodic Elements page where you can get an in-depth view of all the elements,
complete with the industry first side-by-side element comparisons!