*** Advanced Project Notice ***
The following project is an ADVANCED project and may require handling of dangerous materials and/or equipment and is intended to be conducted by adults only!

Purpose
Extract synthetic coloring agents in food products and identify them by paper chromatography.
Additional information
In food industry, coloring of foods is an important aspect. This can be done by a staining process, simple incorporation of dyes or occasionally by technically complex dying procedures. The food coloring agents should be free from toxicity. They should be soluble in water, alcoholic solvents, edible oils or other media of incorporation. Other important characteristics of healthy coloring agents are:
- Freedom from reaction with other components of the food stuff such as alkalis, acids, flavoring materials and preservatives.
- Freedom from attack by bacteria
- Stability to light
Food, drug and cosmetic act in USA has approved seven synthetic dyes for food coloring:
- Allura Red
- Brilliant Blue
- Indigotine
- Fast Green
- Tartrazine
- Sunset Yellow
- Erythrosine
These dyes are stable to heat treatments, pH changes and extended storage conditions. Usually small quantities of food dyes are enough to colorize food products.
With a simple experiment we can extract synthetic colorants from foods, for qualitative analysis. Paper chromatography is an analytical chemistry technique for separating and identifying mixtures that are or can be colored, especially pigments. By this method we will examine whether the extracted colorants are approved ones or not.
Sponsored Links
Required materials
- Sweet pebbles of different colors
- Commercially available standard colorants
- Distilled water
- Liquor ammonia
- Glacial acetic acid
- Analytical balance
- 100ml beaker
- 10cm strip of white wool
- Water bath Bunsen burner
- Whatman no. 3 chromatographic paper (10”x8”)
- Pencil
- Ruler
- Capillary tubes
- A glass chamber
- Developing solvent mixtures:
- n-butanol : acetic acid : water – (20:5:12). Prepare the mixture by adding n-butanol, acetic acid and water in consecutive ratio of 20:5:12. e.g. if you add 20ml of n-butanol then you should add 5ml of acetic acid and 12ml of water to prepare the mixture
- propanol : ammonia – (4:1)
- tri sodium citrate : ammonia : water – (10g : 50ml : 50ml)
Estimated Experiment Time
2 to 3 hours
Step-By-Step Procedure
Extraction of the color from the food
- 1. Separate the sweets into groups according to color.
- 2. Take about 10g sample from one group into 100ml beaker and dissolve it in 50ml distilled water. Likewise dissolve each group separately.
- 3. Acidify the sweet solution with 2-3 drops of glacial acetic acid and put the 10cm long white wool strip into the solution.
- 4. Put the beaker in a water bath. Heat to boil the inner solution of dissolved sweets.
- 5. When it is visible that the wool has colored with colorants from food, remove the wool and wash gently with tap water.
- 6. Transfer the washed wool to another beaker. Add 20ml of distilled water followed by 3-4 drops of liquor ammonia. Boil the content gently.
- 7. Take the wool out of the beaker when the liquid in the beaker has taken up the color of the wool.
- 8. Continue the heating the liquid in beaker to reduce the content volume to near dryness (not to complete dryness but to near dryness).
- 9. Repeat the above extraction procedure with sweet samples of other colors.
Identification of the extracted color by paper chromatography
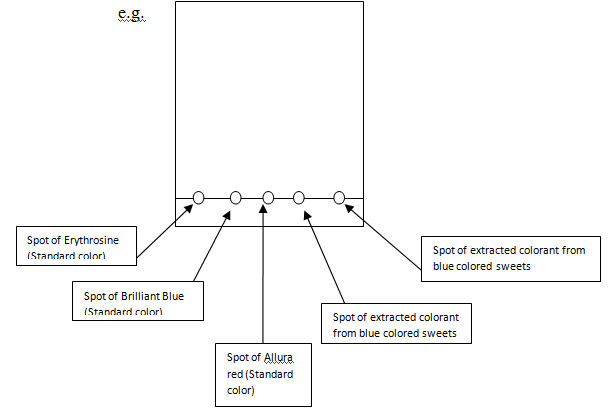
- 1. Take the Whatman no.3 chromatographic paper. Draw a pencil line parallel to the bottom at 2cm distance from the edge of the paper.
- 2. Using a capillary tube, apply a spot of concentrated solution of the unknown color on the pencil line.
- 3. Apply a spot of each standard colorant on pencil line, leaving a 2cm gap between two spots. (see: Figure 1.0)
- 4. Air dry the spots.
- 5. Place the chromatographic paper in a chamber containing one of the above mentioned developing mixtures. Cover the chamber with its lid.
- 6. After solvent front has moved ¾ of the total length of the paper, remove it from the chamber and air dry.
- 7. Compare the distances travelled by the spots of extracted colorants with spots of standard colorants.
Figure 1.0
Note
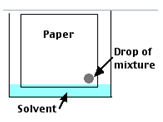
- Dip the paper in mixture as the paper edge barely touches the solvent.
Figure 2.0
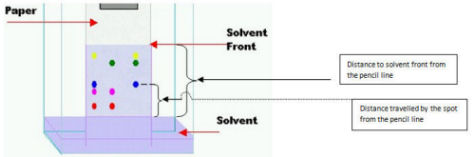
- The lid should properly fit with the chamber and no air leaking holes should exist in the chamber. Leaking atmospheric air inside and diffusion of solvent vapors into atmosphere can cause erroneous results.
- If you place 2 spots of same colorant in the same chromatographic paper, the distance they travel within same period of time is similar. If the spots are placed in different chromatographic papers then you have to compare the Rf values to determine whether the spots contain same compound or different compounds. (Rf = distance travelled by the spot from the pencil line divided by the distance to solvent front from the pencil line). If any spot contains a mixture of colorants then it separates into several spots; each containing one component.
Figure 3.0
Observation
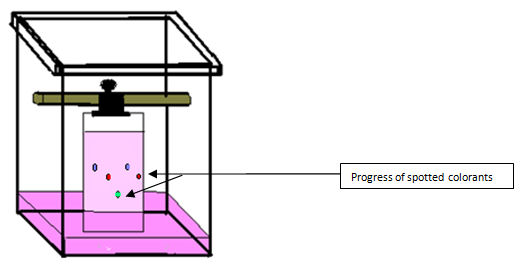
You will observe the progress of spots as in figure 4.0 above.
By comparing the distances travelled by colorants extracted from sweets with the spots of standard colorants, you can check whether those sweets contain standard colorants.
If the extracted solution contains a mixture of standard colorants you observe the separation of components as in figure 5.0 below.
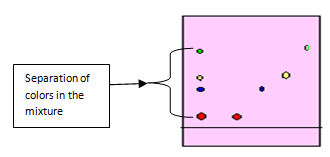
Result
According to your observations you can identify the synthetic colorants in tested sweets. Perform this experiment with well known brands. If you find that the extracted colorants do not match with the standard colorants perform the chromatography again before jump into a quick decision.
In paper chromatography the solvent in the pool at the bottom of the vessel (in which the paper is dipped) rises up the paper by capillary action against the force of gravity. Spots of colorants travel along the solvent but due to different molecular structures of different compounds the travelling rates are different.
The capillary action occurs as a result of the attraction of the solvent molecules to the paper,also this can be explained as differential adsorption of the solute components into the solvent. As the solvent rises through the paper it meets and dissolves the sample mixture, which will then travel up the paper with the solvent solute sample. Different compounds in the sample mixture travel at different rates due to differences in solubility in the solvent, and due to differences in their attraction to the fibers in the paper. The more soluble the component the further it goes. Paper chromatography takes anywhere from several minutes to several hours. In some cases, paper chromatography does not separate pigments completely; this occurs when two substances appear to have the same values in a particular solvent. In these cases, two-way chromatography is used to separate the multiple-pigment spots.
Sponsored Links
Take a moment to visit our table of Periodic Elements page where you can get an in-depth view of all the elements,
complete with the industry first side-by-side element comparisons!